Rapid Construction of Structurally Diverse Quinolizidines, Indolizidines, and Their Analogues via Ruthenium‐Catalyzed Asymmetric Cascade Hydrogenation/Reductive Amination
Ya Chen, Yan-Mei He, Shanshan Zhang, Tingting Miao, and Qing-Hua Fan*
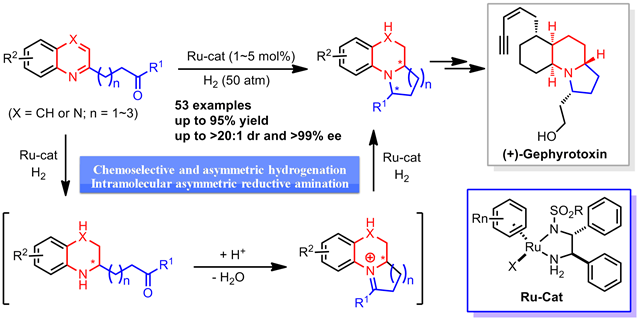
A rapid construction of enantioenriched benzo‐fused quinolizidines, indolizidines, and their analogues by ruthenium‐catalyzed asymmetric cascade hydrogenation/reductive amination of quinolinyl‐ and quinoxalinyl‐containing ketones has been developed. This reaction proceeds under mild reaction conditions, affording chiral benzo‐fused aliphatic N‐heterocyclic compounds with structural diversity in good yields (up to 95 %) with excellent diastereoselectivity (up to >20:1 dr) and enantioselectivity (up to >99 % ee). Furthermore, this catalytic protocol is applicable to the formal synthesis of (+)‐gephyrotoxin.
This work has been published in Angew. Chem. Int. Ed., 2019, 58, 3809-3813 (back cover).

