Iron Carbonyl Catalyzed Redox-Neutral [4+2] Annulation of N?H Imines and Internal Alkynes via C?H Bond Activation
Teng Jia, Chongyang Zhao, Ruoyu He, Hui Chen,* and Congyang Wang*
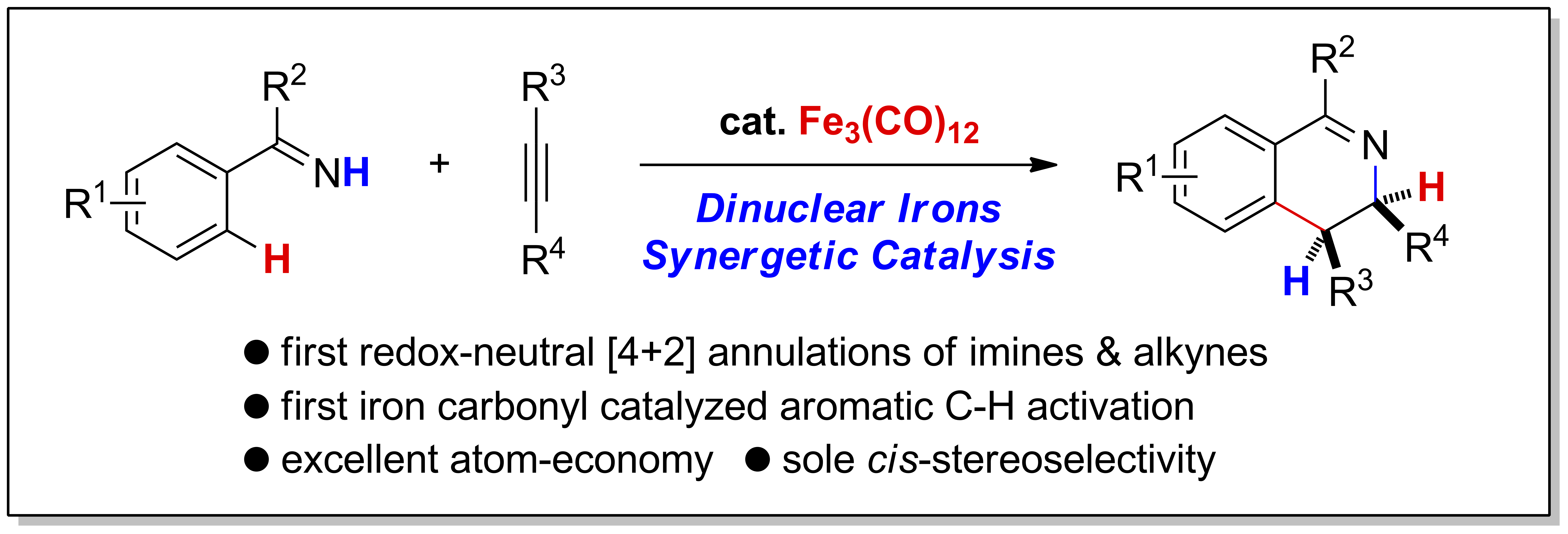
Stoichiometric C−H bond activation of arenes mediated by iron carbonyls was reported by Pauson as early as in 1965, the catalytic C−H transformations based on which haven’t been developed yet. Herein, an iron-catalyzed annulation of N-H imines and internal alkynes to furnish cis-3,4-dihydroisoquinolines were described, which represents the first iron carbonyl catalyzed C−H activation reaction of arenes. Remarkablely, this is also the first redox-neutral [4+2] annulation of imines and alkynes via C−H activation. The reaction also features sole cis-stereoselectivity and excellent atom-economy whereby no base, external ligand or additive is required. Experimental and theoretical studies revealed an oxidative addition mechanism for C-H bond activation affording a dinuclear ferracycle and a synergetic diiron-promoted H-transfer to alkyne as the turnover-determining step.
This work has been published in Angew. Chem., Int. Ed. 2016, 55, 5268-5271.

