Research Progress
-
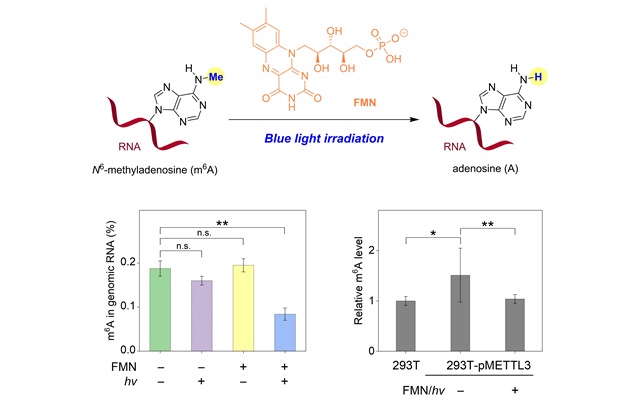
Identification of Flavin Mononucleotide as a Cell‐Active Artificial N6‐Methyladenosine RNA Demethylase
N6‐Methyladenosine (m6A) represents a common and highly dynamic modification in eukaryotic RNA that affects various cellular pathways. Natural dioxygenases such as FTO and ALKBH5 are enzymes that demethylate m6A residues in mRNA. Herein, the first identification of a small‐molecule modulator that functions as an artificial m6A demethylase is reported. Flavin mononucleotide (FMN), the metabolite produced by riboflavin kinase, mediates substantial photochemical demethylation of m6A residues of RNA ...
-
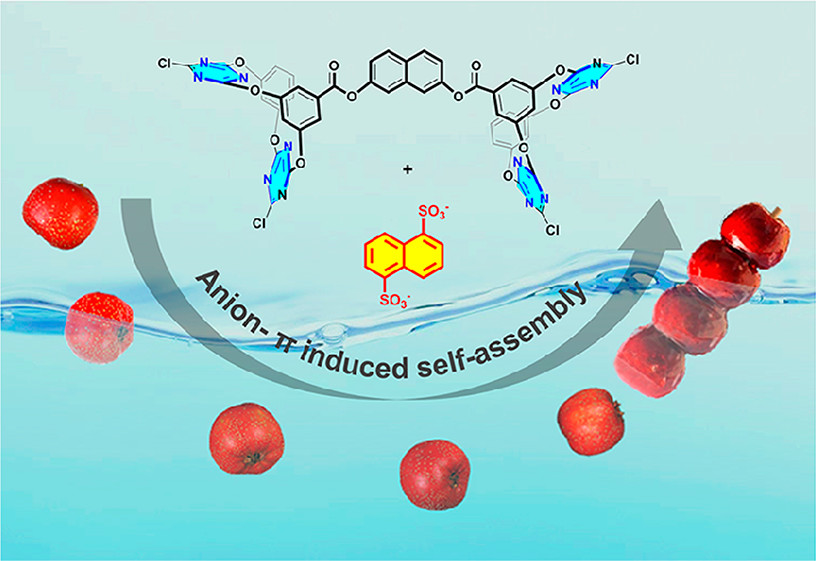
Toward Anion?π Interactions Directed Self-Assembly with Predesigned Dual Macrocyclic Receptors and Dianions
Realizing anion?π interaction induced self-assembly with charge-neutral π receptors as building components is extremely challenging. We designed and synthesized a series of bisoxacalix[2]arene[2]triazines 7–11 in which two macrocyclic motifs are linked in diverse branching angle and rigidity. Crystal structures showed the use of rigid linkers is able to control the orientation of the two macrocyclic cavities. The interplay between the two cavities was revealed by binding studies of 8–11 with chl ...
-
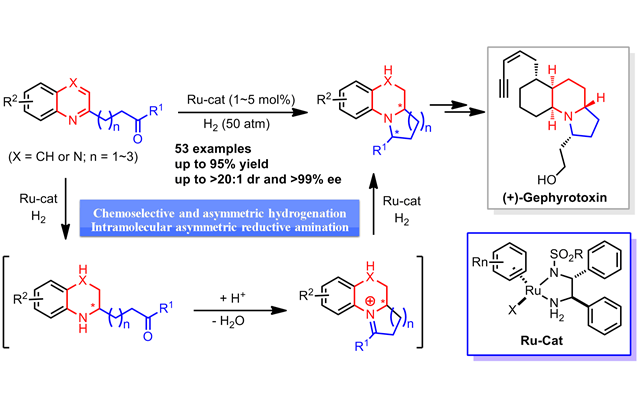
Rapid Construction of Structurally Diverse Quinolizidines, Indolizidines, and Their Analogues via Ruthenium‐Catalyzed Asymmetric Cascade Hydrogenation/Reductive Amination
A rapid construction of enantioenriched benzo‐fused quinolizidines, indolizidines, and their analogues by ruthenium‐catalyzed asymmetric cascade hydrogenation/reductive amination of quinolinyl‐ and quinoxalinyl‐containing ketones has been developed. This reaction proceeds under mild reaction conditions, affording chiral benzo‐fused aliphatic N‐heterocyclic compounds with structural diversity in good yields (up to 95?%) with excellent diastereoselectivity (up to >20:1 dr) and enantioselectivity ( ...
-
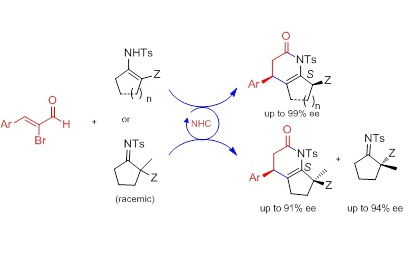
(Dynamic) Kinetic Resolution of Enamines/Imines: Enantioselective N‐Heterocyclic Carbene Catalyzed [3+3] Annulation of Bromoenals and Enamines/Imines
The enantioselective N‐heterocyclic carbene catalyzed [3+3] annulation of α‐bromoenals by dynamic kinetic resolution (DKR) of enamines and normal resolution of α,α‐disubstituted imines were developed. The corresponding substituted dihydropyridones were isolated in good yields with excellent diastereo‐ and enantioselectivities, and a high selective factor (up to 83) was realized for the resolution of α,α‐disubstituted imines.
-
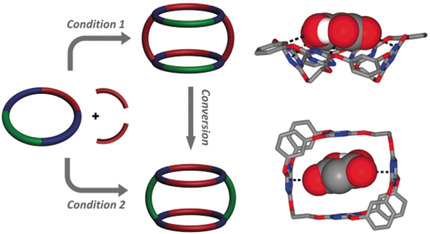
Diversity-Oriented Construction and Interconversion of Multicavity Supermacrocycles for Cooperative Anion-π Binding
A one-pot strategy for the diverse construction of a series of supermacrocycles was realized using rationally designed macrocyclic precursors. The base was found to have a significant effect not only on the size distribution but also on the structure of the supermacrocycles formed. While the use of less CsF (< 4.0 equiv) afforded regular supermacrocycles containing up to four macrocyclic precursor subunits, the use of more CsF (> 8.0 equiv) resulted in structurally reorganized supermacrocyles fe ...
-
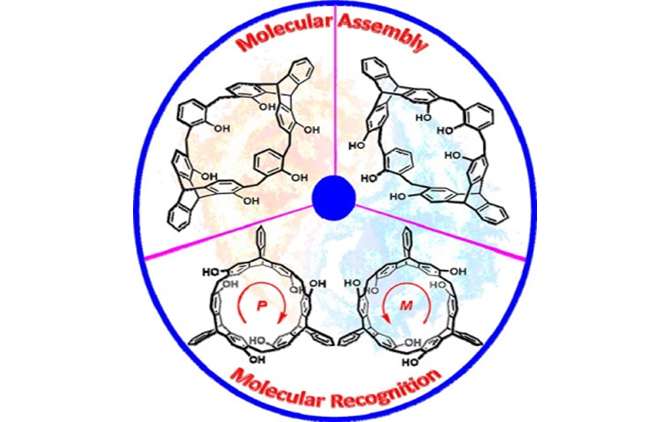
Triptycene-Derived Macrocyclic Arenes: From Calixarenes to Helicarenes
This Account summarizes our recent research results on the synthesis and structures of the triptycene-derived macrocyclic arenes and analogues and their applications in host?guest chemistry and molecular assembly. We believe that these macrocyclic arenes, especially helicarenes, could be utilized as new synthetic hosts and find wide potential applications in macrocyclic and supramolecular chemistry.
-
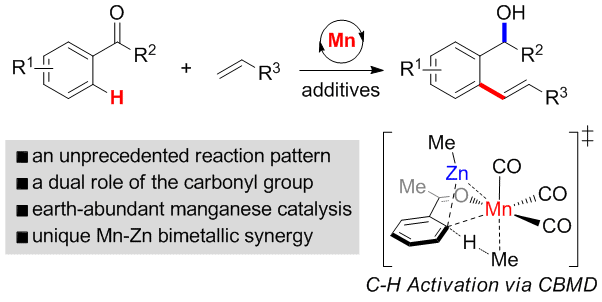
Manganese-Catalyzed Redox-Neutral C-H Olefination of Ketones with Unactivated Alkenes
Since 1987, stoichiometric cyclomanganation of ketones and following reactions with olefins in the presence of palladium salts or trimethylamine N-oxide (Me3N+O–) had been reported, while the catalytic versions remain untouched so far. Herein, the first manganese-catalyzed redox-neutral C-H olefination of ketones with unactivated alkenes is described, which shows a distinct reactivity with its parent stoichimetric reactions. Remarkably, mechanistic experiments and DFT calculations uncovers a uni ...
-
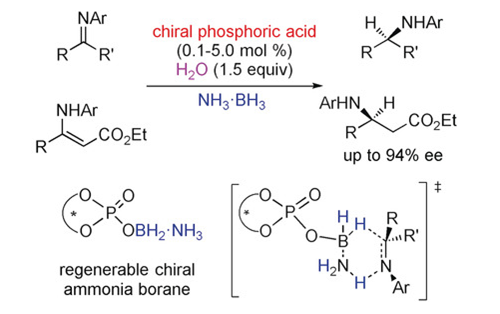
A Continuously Regenerable Chiral Ammonia Borane for Asymmetric Transfer Hydrogenations
A novel chiral ammonia borane was designed and developed through the dehydrogenation of ammonia borane with a chiral phosphoric acid, which was highly effective for the asymmetric transfer hydrogenation of imines and b-enamino esters to afford high levels of reactivities and enantioselectivities. Significantly, this chiral ammonia borane can be continuously regenerated during the transfer hydrogenation with the assistance of water and ammonia borane, which made it possible to obtain satisfactory ...
-
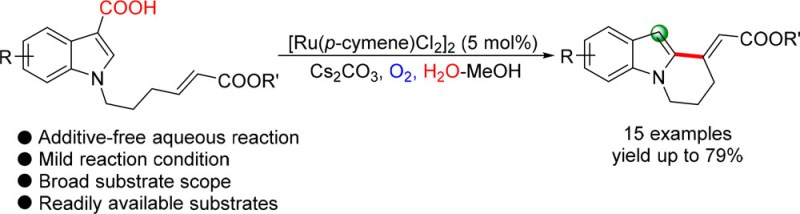
Ruthenium-Catalyzed Decarboxylative C-H Alkenylation in Aqueous Media: Synthesis of Tetrahydropyridoindoles
We disclose herein a Ru(II)-catalyzed decarboxylative and oxidative coupling of N-substituted indolyl carboxylic acids with broad substrate scope in an aqueous solution. This method provides a sustainable and efficient access to synthesize various indole-fused cyclohexanyl acetic acids under mild conditions.
-
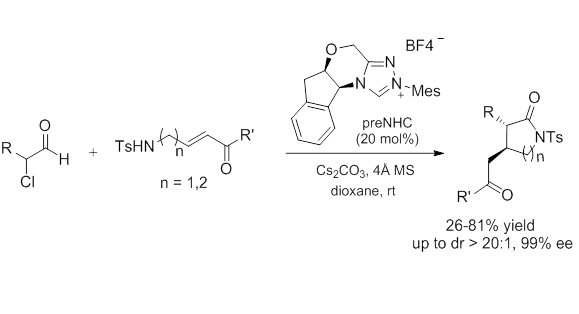
N-Heterocyclic Carbene-Catalyzed Annulation of alpha-Chloroaldehydes with gamma-/delta-Amino-alpha,beta-Unsaturated Ketones: Enantioselective Synthesis of Pyrrolidones and Piperidones.
Pyrrolidones and Piperidones: The N-heterocyclic carbene-catalyzed [2+3] and [2+4] annulations of a-chloroaldehdydes with - or -amino-α,β-unsaturated ketones were developed to give 3,4-disubstituted pyrrolidones and piperidones in good yields with exclusive trans-selectivities and excellent enantioselectivities.
-
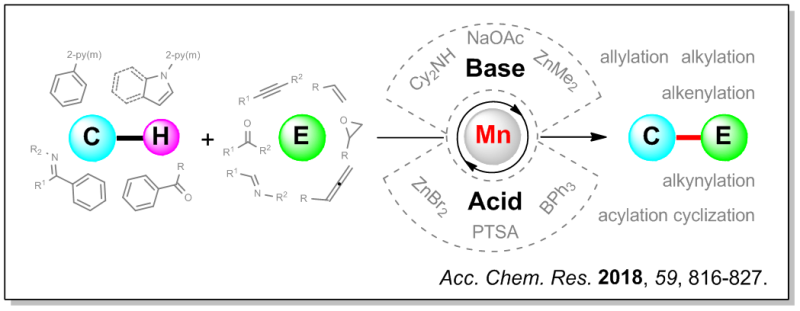
Inert C-H Bond Transformations Enabled by Organometallic Manganese Catalysis
Traditional organic synthesis relies heavily on the transformations of various preinstalled functional groups, such as cross-coupling reactions using organohalides and organometallic reagents. The strategy of C–H activation enables the direct formation of C–C/C–X (X = heteroatom) bonds from inert C–H bonds, which can enhance the atom- and step-economy of organic synthesis. To date, precious metals have overwhelmingly dominated the C–H activation field; however, the rarity and high cost of these ...
-
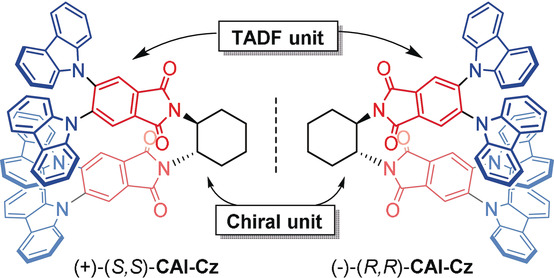
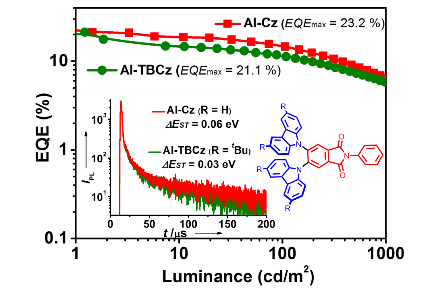
Stable Enantiomers Displaying Thermally Activated Delayed Fluorescence: Efficient OLEDs with Circularly Polarized Electroluminescence
Aromatic‐imide‐based thermally activated delayed fluorescent (TADF) enantiomers, (+)‐(S,S)‐CAI‐Cz and (?)‐(R,R)‐CAI‐Cz, were efficiently synthesized by introducing a chiral 1,2‐diaminocyclohexane to the achiral TADF unit. The TADF enantiomers exhibited high PLQYs of up to 98?%, small ΔEST?values of 0.06?eV, as well as obvious temperature‐dependent transient PL spectra, thus demonstrating their excellent TADF properties. Moreover, the TADF enantiomers showed mirror‐image CD and CPL activities. No ...
-
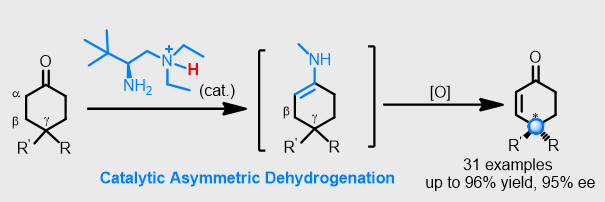
Catalytic Desymmetrizing Dehydrogenation of 4-Substituted Cyclohexanones through Enamine Oxidation
A desymmetrizing dehydrogenation process catalyzed by a chiral primary amine is described herein. The reaction proceeds through the oxidation of a ketone enamine by IBX and enables the highly enantioselective desymmetrization of 4‐substituted cyclohexanones with the generation of chiral 4‐substituted cyclohexenones containing a remote γ‐stereocenter.
-
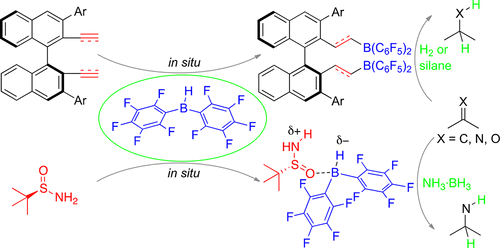
Frustrated Lewis Pairs Catalyzed Asymmetric Metal-Free Hydrogenations and Hydrosilylations
The use of frustrated Lewis pairs is an extremely important approach to metal-free hydrogenations. In contrast to the rapid growth of catalytic reactions, asymmetric hydrogenations are far less developed due to a severe shortage of readily available chiral frustrated Lewis pair catalysts with high catalytic activities and selectivities. Unlike the stable Lewis base component of frustrated Lewis pairs, the moisture-sensitive boron Lewis acid component is difficult to prepare. The development of c ...
-
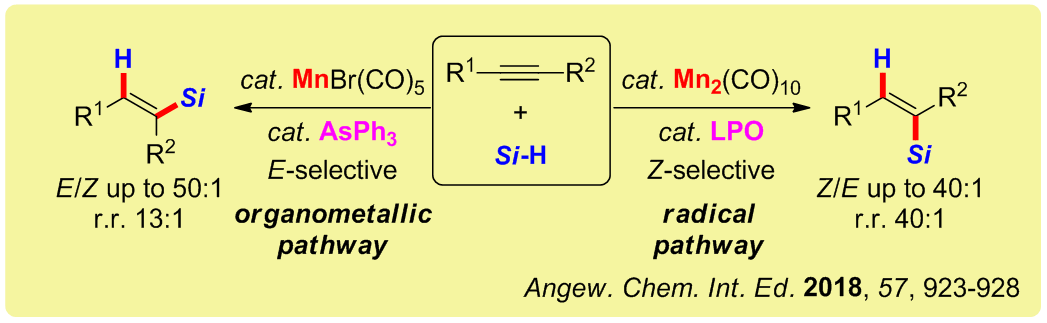
Dichotomy of Manganese Catalysis via Organometallic or Radical Mechanism: Stereodivergent Hydrosilylation of Alkynes
Herein, we disclose the first manganese‐catalyzed hydrosilylation of alkynes featuring diverse selectivities. The highly selective formation of E‐products was achieved by using mononuclear MnBr(CO)5 with the arsenic ligand, AsPh3. Whereas using the dinuclear catalyst Mn2(CO)10 and LPO (dilauroyl peroxide) enabled the reversed generation of Z‐products in good to excellent stereo‐ and regioselectivity. Such a way of controlling the reaction stereoselectivity is unprecedented. Mechanistic experimen ...
-
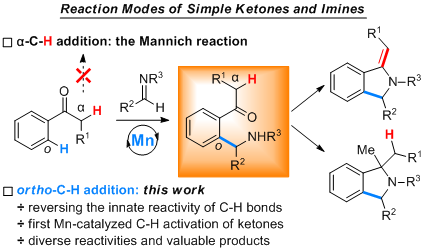
Aromatic C-H Addition of Ketones to Imines Enabled by Manganese-Catalysis
Selectivity control of varied C-H bonds in a complex molecule is a long-standing goal and still a great challenge in C-H activation field. Most often, such selectivity is achieved by the innate reactivity of different C-H bonds. In this context, the classic Mannich reaction of acetophenone derivatives and imines is ascribed to the more reactive C(sp3)-H bonds α to the carbonyl, with the much less reactive aromatic C(sp2)-H bonds remaining intact. Herein we report an aromatic C(sp2)-H addition of ...
-
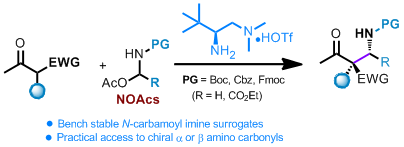
Catalytic Asymmetric Mannich Reaction with N-Carbamoyl Imine Surrogates of Formaldehyde and Glyoxylate
N, O-acetals (NOAcs) have been developed as bench stable surrogates for N-carbamoyl (Boc, Cbz and Fmoc) formaldehyde and glyoxylate imines in asymmetric Mannich reactions. The NOAcs can be directly utilized in the chiral primary amine catalyzed Mannich reactions of both acyclic and cyclic β-ketocarbonyls with high yields and excellent stereoselectivity. The current reaction offers a straightforward approach in the asymmetric synthesis of α- or β-amino carbonyls bearing chiral quaternary centers ...
-
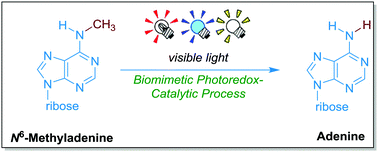
Visible-light-mediated oxidative demethylation of N6-methyl adenines
We report a simple protocol that affords oxidative demethylation of N6-methyl groups in N6-methyl adenines (m6A). The biologically compatible photocatalyst riboflavin prompts a highly selective C–H abstraction from N6-methyl in adenines under the irradiation of a visible blue LED light, affording a novel and highly selective biomimetic demethylation of m6A and related N-methyl adenine analogues.
-
Catalytic Regio- and Enantioselective [4+2] Annulation Reactions of Non-activated Allenes by a Chiral Cationic Indium Complex
A highly enantioselective and regioselective [4+2] annulation reaction of β, γ-unsaturated ?-ketoesters with non-activated terminal and 1,1-disubstituted allenes was developed. The identification of a chiral cationic indium/phosphate complex is the key to achieve the current reaction. The reaction affords the corresponding C3-selective dihydropyrans in good yields and with high enantioselectivities (up to 99% ee).
-
Aromatic Imide Based Thermally Activated Delayed Fluorescence Materials for Highly Efficient Organic Light Emitting Diodes
A new kind of aromatic imide-based thermally activated delayed fluorescence (TADF) materials with twisted donor-acceptor-donor skeleton were efficiently synthesized, which exhibited excellent thermal stability and high photoluminescence quantum yields. The small ?EST (< 0.1 eV) along with the obvious temperature dependent delayed component of transient photoluminescence (PL) spectra demonstrated their excellent TADF properties. Moreover, the performances of organic light emitting diodes using TA ...