Research Progress
-
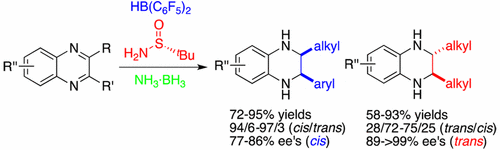
Asymmetric Transfer Hydrogenations of 2, 3-Disubstituted Quinoxalines with Ammonia Borane
An asymmetric transfer hydrogenation of 2,3-disubstituted quinoxalines using a chiral frustrated Lewis pair of Piers’ borane and (R)-tert-butylsulfinamide as the catalyst with ammonia borane as the hydrogen source has been successfully realized. For 2-alkyl-3-arylquinoxaline substrates, cis-tetrahydroquinoxalines were obtained as the predominant products in high yields with 77–86% ee. In contrast, trans isomers were often furnished as major products for the reactions of 2,3-dialkylquinoxalines w ...
-
Asymmetric Hydrogenation of In?Situ Generated Isochromenylium Intermediates by Copper/Ruthenium Tandem Catalysis
The first asymmetric hydrogenation of in situ generated isochromenylium derivatives is enabled by tandem catalysis with a binary system consisting of Cu(OTf)2 and a chiral cationic ruthenium–diamine complex. A range of chiral 1H-isochromenes were obtained in high yields with good to excellent enantioselectivity. These chiral 1H-isochromenes could be easily transformed into isochromanes, which represent an important structural motif in natural products and biologically active compounds. The chira ...
-
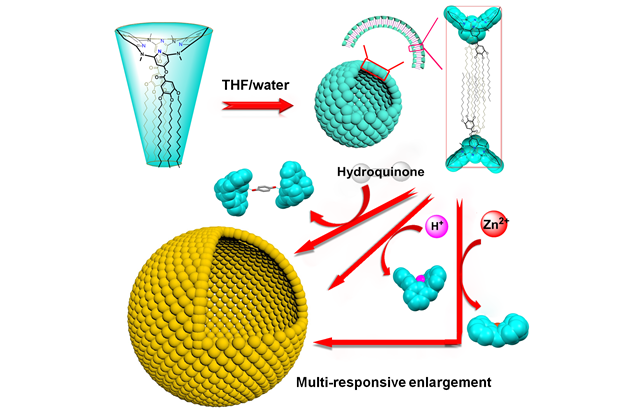
Multi-responsive Vesicles Composed of Amphiphilic Azacalix[4]pyridine Derivatives
Bio-mimicry of multi-responsive recognition of cell membrane with artificial membranes is challengeable. In this work, we designed azacalix[4]pyridine-based amphiphilic molecules 1 and 2. The self-assembly behaviours of 1 and 2 were investigated in aqueous medium. As demonstrated by DLS, SEM, TEM and LSCM measurements, 1 formed stable vesicles (size 322 nm) in a mixture of THF/water whereas 2 produced giant vesicles with decreased stability (size 928 nm). The vesicles composed of 1, with surface ...
-
Ruthenium-Catalyzed Enantioselective Phenanthridine Derivatives
The first asymmetric hydrogenation of phenanthridines catalyzed by chiral cationic ruthenium diamine complexes has been developed with up to 92% ee and full conversions. The choice of the counteranion of the catalyst was found to be critical for achieving high enantioselectivity. In addition, the obtained 5,6-dihydrophenanthridine could be used as a chiral hydride donor for organocatalytic asymmetric transfer hydrogenation.
-
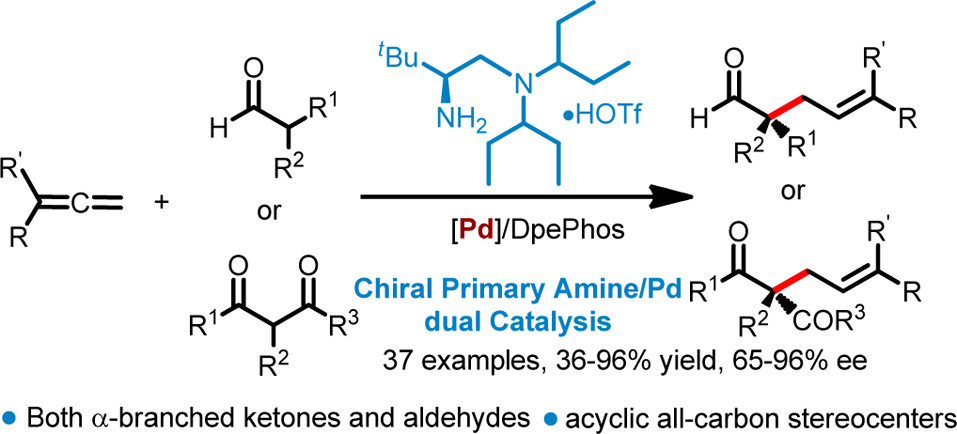
Enantioselective Terminal Addition to Allenes by Dual Chiral Primary Amine/Palladium Catalysis
Nucleophilic addition to allenes is one of the most atom-economic approaches for the synthesis of functionlized allylic compounds. A dual chiral primary amine and palladium catalyzed enantioselective terminal addition to allenes with ?-branched β-ketocarbonyls and aldehydes. The reactions affords allylic adducts bearing acyclic all-carbon quaternary centers with high regio and enantioselectivity. A wide range of allenes including those aliphatic or 1,1’-disubstituted could be employed, thus expa ...
-
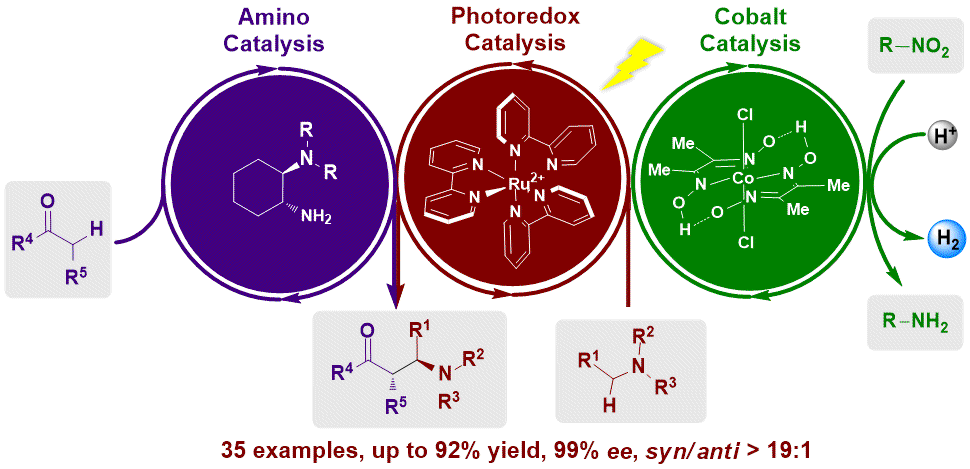
Visible Light Promoted Asymmetric Cross-Dehydrogenative Coupling of Tertiary Amines to Ketones by Synergistic Multiple Catalysis
An unprecedented catalytic asymmetric cross-dehydrogenative coupling reaction between tertiary amines and simple ketones via tricatalytic cycles under visible light irradiation. The process is enabled by a simple chiral primary amine catalyst through the coupling of a catalytic enamine intermediate and a iminum intermediate in situ generated from tetrahydroisoquinoline derivatives via coupled Ru/Co catalysis.
-
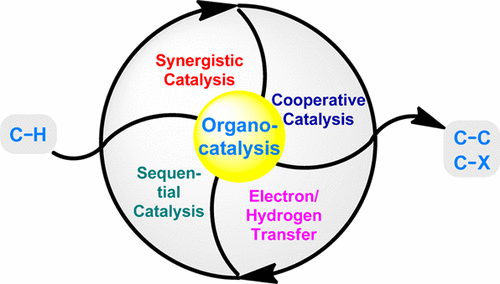
Organocatalysis in Inert C?H Bond Functionalization
As two coexisting and fast-growing research fields in modern synthetic chemistry, the merging of organocatalysis and C?H bond functionalization is well foreseeable, and the joint force along this line has been demonstrated to be a powerful approach in making inert C?H bond functionalization more viable, predictable, and selective. In this review, we provide a comprehensive summary of organocatalysis in inert C?H bond functionalization over the past two decades. The review is arranged by types of ...
-
Transition-Metal-Free Alkynylation of 2?Oxindoles through Radical-Radical Coupling
An effective transition-metal-free approach for the synthesis of 3-alkynyl-2-oxindoles through a radical?radical coupling process was developed. The reaction was general with respect to 2-oxindoles and iodoalkynes and provided the desired products bearing a quaternary center at C3 in good to excellent yields, making this method synthetically viable and attractive for the synthesis of spiro and fused 2-oxindole derivatives.
-
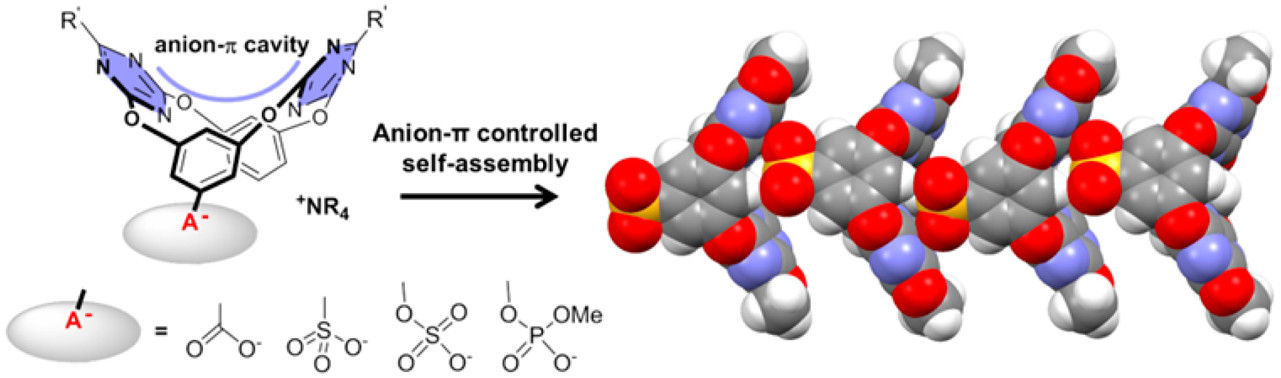
Anionic head containing oxacalix[2]arene[2]triazines: Synthesis and anion?π-directed self-assembly in solution and solid State
A series of oxacalix[2]arene[2]triazines bearing one anionic head such as carboxylate, sulfonate, sulfate, and phosphate were synthesized. With the anionic head and complementary V-shape electron-deficient cavity, these macrocycles can serve as dual building units, and their anion?π directed self-assembly was investigated. The formation of oligomeric aggregates in solution was revealed by nuclear magnetic resonance, dynamic light scattering, and mass spectroscopy. Crystal structures further conf ...
-
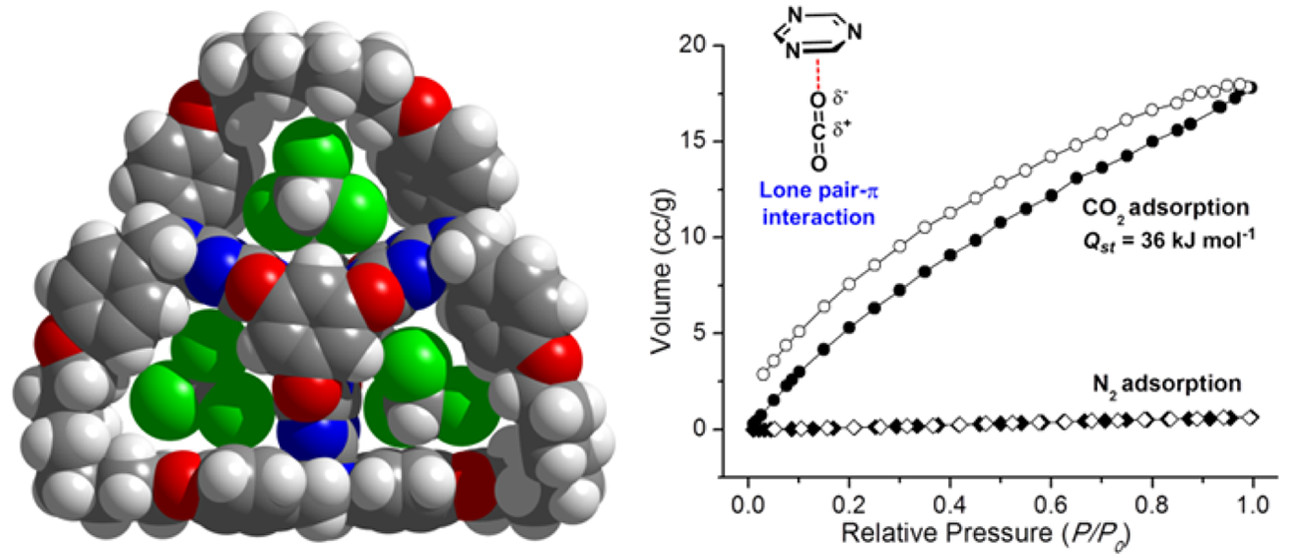
Molecular Barrel by a Hooping Strategy: Synthesis, Structure, and Selective CO2 Adsorption Facilitated by Lone Pair?π Interactions
A sophisticated molecular barrel was efficiently constructed by hooping a 63 membered loop around a D3h-symmetric, shape-persistent bis(tetraoxacalix[2]arene[2]triazine) core. The hooping strategy involved threefold ring-closing metathesis (RCM) reactions of six branched olefin arms which were pre-anchored on the inner core. Through hooping, the loop tightens the cage structure and significantly enhances its stability towards nucleophilic decomposition. X-ray crystal structure showed the molecul ...
-
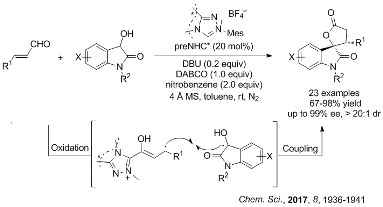
N-Heterocyclic carbene-catalyzed oxidative cross coupling of homoenolate and enolate
Being a step- and atom-economical process via C–H functionalization, the oxidative cross coupling reaction is of great value in modern organic synthesis. The oxidative coupling of two enolates is a powerful route to 1,4-dicarbonyl compounds, which has been well established. However, the oxidative cross coupling of homoenolate and enolate remains unexplored but is highly desired for the construction of 1,5-dicarbonyl and related compounds. Recently, the reaserchers in LMRF for the first time dev ...
-
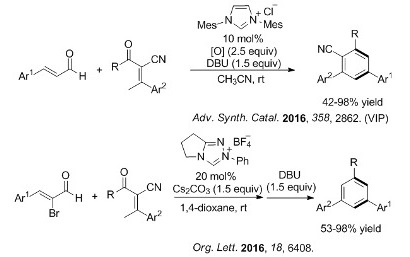
N-Heterocyclic carbene-catalyzed synthesis of multi-substituted benzenes
Multi-substituted benzene is a ubiquitous and important structural unit found in various natural products, advanced functional materials and ligands of organometallic compounds. Among the various types of multi-substituted benzenes, the 1,3,5-trisubstituted ones are of great importance for their diverse applications in material science.Recently, The researchers in LMRF developed an oxidative NHC-catalyzed [2+4] annulation of enals and α-cyano-β-methylenones for the synthesis of multi-substituted ...
-
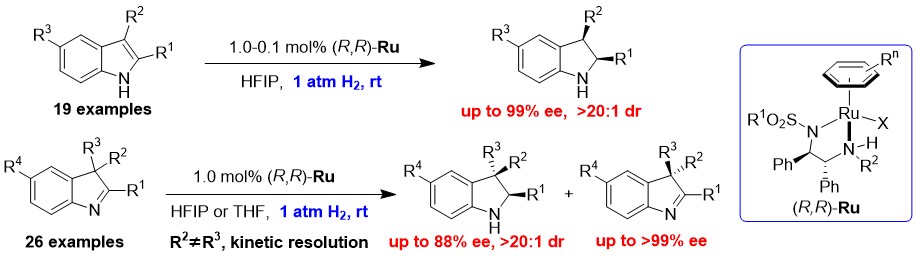
Highly Enantioselective Synthesis of Indolines: Asymmetric Hydrogenation at Ambient Temperature and Pressure with Cationic Ruthenium Diamine Catalysts
A highly enantioselective synthesis of indolines by asymmetric hydrogenation of 1H-indoles and 3H-indoles at ambient temperature and pressure, catalyzed by chiral phosphine-free cationic ruthenium complexes, has been developed. Excellent enantio- and diastereoselectivities (up to > 99% ee, > 20:1 d.r.) were obtained for a wide range of indole derivatives, including unprotected 2-substituted and 2,3-disubstituted 1H-indoles, as well as 2-alkyl- and 2-aryl-substituted 3H-indoles.
-
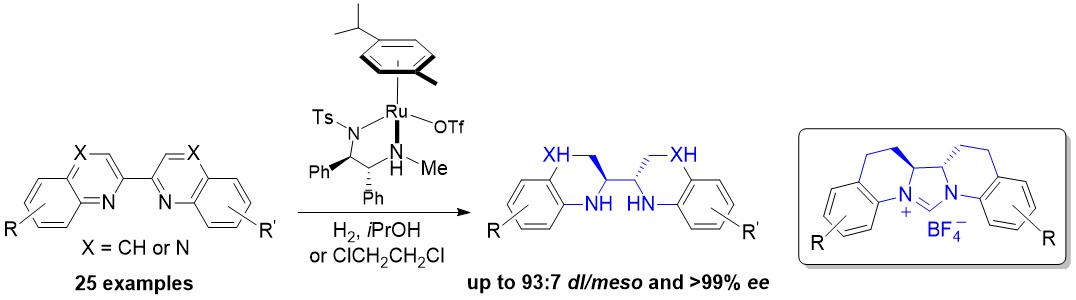
Highly Enantioselective Direct Synthesis of Endocyclic Vicinal Diamines through Chiral Ru(diamine)-Catalyzed Hydrogenation of 2,2′-Bisquinoline Derivatives
An asymmetric hydrogenation of 2,2′-bisquinoline and bisquinoxaline derivatives, catalyzed by chiral cationic ruthenium diamine complexes, was developed. A broad range of chiral endocyclic vicinal diamines were obtained in high yields with excellent diastereo- and enantioselectivity (up to 93:7 dl/meso and >99?% ee). These chiral diamines could be easily transformed into a new class of chiral N-heterocyclic carbenes (NHCs), which are important but difficult to access.
-
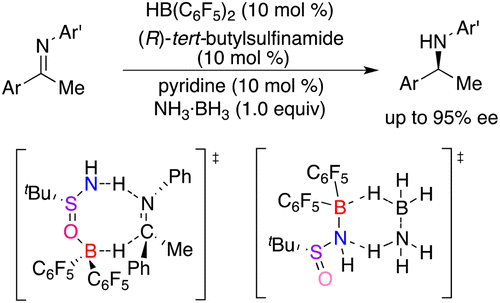
A Frustrated Lewis Pair Mimic Catalyzed Asymmetric Transfer Hydrogenation of Imines Using Ammonia Borane
Inspired by the zwitterion species generated from the splitting of H2 by frustrated Lewis pairs, we put forward a novel frustrated Lewis pair by the combination of Hδ- and Hδ+ incorporated Lewis acid and base together. Piers’ borane and chiral tert-butylsulfinamide were chosen as the FLP, and a metal-free asymmetric transfer hydrogenation of imines was realized with high enantioselectivities. Significantly, with ammonia borane as hydrogen source, a catalytic asymmetric reaction using 10 mol % of ...
-
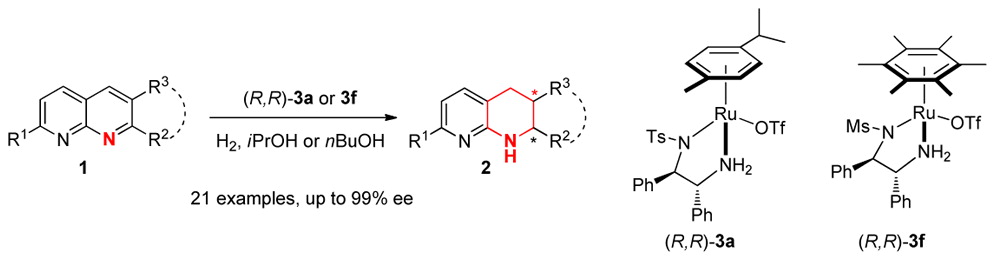
Ruthenium-Catalyzed Enantioselective Hydrogenation of 1,8-Naphthyridine Derivatives
The first asymmetric hydrogenation of 2,7-disubstituted 1,8-naphthyridines catalyzed by chiral cationic ruthenium diamine complexes has been developed. A wide range of 1,8-naphthyridine derivatives were effectively hydrogenated to give 1,2,3,4-tetrahydro-1,8-naphthyridines with up to 99% ee and full conversions. The method provides a practical and facile approach to the preparation of valuable chiral heterocyclic building blocks and useful motifs for a new kind of P,N-ligands.
-
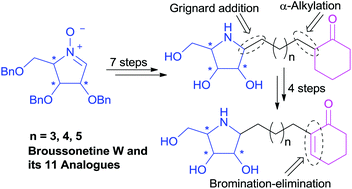
First total synthesis of (+)-broussonetine W: glycosidase inhibition of natural product & analogs
The first total synthesis of (+)-broussonetine W (4), a naturally-occurring pyrrolidine iminosugar isolated from the traditional Chinese medical plant Broussonetia kazinoki SIEB (Moraceae), has been completed through a concise synthetic route starting from the readily available D-arabinose derived cyclic nitrone 10 in 11 steps and 31% overall yield, with regioselective installation of the α,β-unsaturated ketone functional group by the elimination of HBr from α-bromoketone as the key step. A numb ...
-
N-Heterocyclic Carbene-Catalyzed [3 + 4] Annulation of Enals and Alkenyl Thiazolones: Enantioselective Synthesis of Thiazole-fused ε-Lactones
The bifunctional N-heterocyclic carbene catalyzed [3 + 4] annulation of enals and 5-alkenyl thiazolones was developed, giving the corresponding thiazole-fused ?-lactones in high yields with excellent diastereoselectivties and enantioselectivities. The thiazole-fused ?-lactone could be isomerized to the spirocyclic thiazolone-cyclopentanone without erosion of enantioselectivity.
-
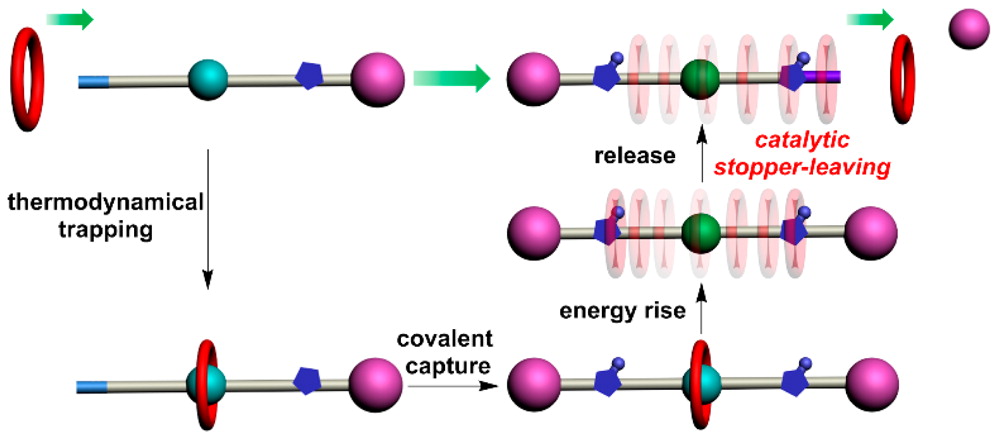
Directional Molecular Transportation Based on a Catalytic Stopper-Leaving Rotaxane System
Ratchet mechanism has proved to be a key principle in designing molecular motors and machines that exploit random thermal fluctuations for directional motion with energy input. To integrate ratchet mechanism into artificial systems, precise molecular design is prerequisite to control the pathway of relative motion between their subcomponents, which is still formidable challenge. Herein, we report a straightforward method to control the transportation barrier of a macrocycle by selectively detach ...
-
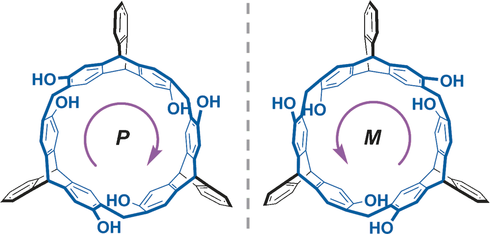
Triptycene-Based Chiral Macrocyclic Hosts for Highly Enantioselective Recognition of Chiral Guests Containing a Trimethylamino Group
A new class of chiral macrocyclic arene composed of three chiral 2,6-dihydroxyltriptycene subunits bridged by methylene groups was designed and synthesized. Structural studies showed that the macrocyclic molecule adopts a hex-nutlike structure with a helical chiral cavity and highly fixed conformation. Efficient resolution was achieved through the introduction of chiral auxiliaries to give a couple of enantiopure macrocycles, which exhibited high enantioselectivity towards three pairs of chiral ...