First total synthesis of (+)-broussonetine W: glycosidase inhibition of natural product & analogs
Ying-Ying Song, Kyoko Kinami, Atsushi Kato,* Yue-Mei Jia, Yi-Xian Li, George W. J. Fleet and Chu-Yi Yu*
The first total synthesis of (+)-broussonetine W (4), a naturally-occurring pyrrolidine iminosugar isolated from the traditional Chinese medical plant Broussonetia kazinoki SIEB (Moraceae), has been completed through a concise synthetic route starting from the readily available D-arabinose derived cyclic nitrone 10 in 11 steps and 31% overall yield, with regioselective installation of the α,β-unsaturated ketone functional group by the elimination of HBr from α-bromoketone as the key step. A number of analogs of (+)-broussonetine W (4) with variable side chain length, different polyhydroxylated pyrrolidine core configurations or saturated cyclohexanones have also been prepared to explore the glycosidase inhibition and the preliminary structure–activity relationship of this intriguing class of compounds. Glycosidase inhibition studies identified the natural product (+)-broussonetine W (4) as a selective and potent inhibitor of β-galactosidase (IC50 = 0.03 μM), while its enantiomer was a selective and potent inhibitor of α-glucosidase (IC50 = 0.047 μM). It was found that the configuration of the polyhydroxylated pyrrolidine ring played a key role on their glycosidase inhibitory activities. The length of side chain and α, β-unsaturated ketone functional group also exhibited some effect on their glycosidase inhibition.
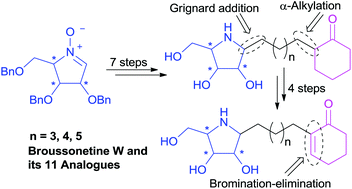
This work has been published in Org. Biomol. Chem. 2016, 14, 5157-5174.

