Highly Enantioselective Direct Synthesis of Endocyclic Vicinal Diamines through Chiral Ru(diamine)-Catalyzed Hydrogenation of 2,2′-Bisquinoline Derivatives
Wenpeng Ma+ , Jianwei Zhang+ , Cong Xu, Fei Chen, Yan-Mei He, and Qing-Hua Fan*
An asymmetric hydrogenation of 2,2′-bisquinoline and bisquinoxaline derivatives, catalyzed by chiral cationic ruthenium diamine complexes, was developed. A broad range of chiral endocyclic vicinal diamines were obtained in high yields with excellent diastereo- and enantioselectivity (up to 93:7 dl/meso and >99 % ee). These chiral diamines could be easily transformed into a new class of chiral N-heterocyclic carbenes (NHCs), which are important but difficult to access.
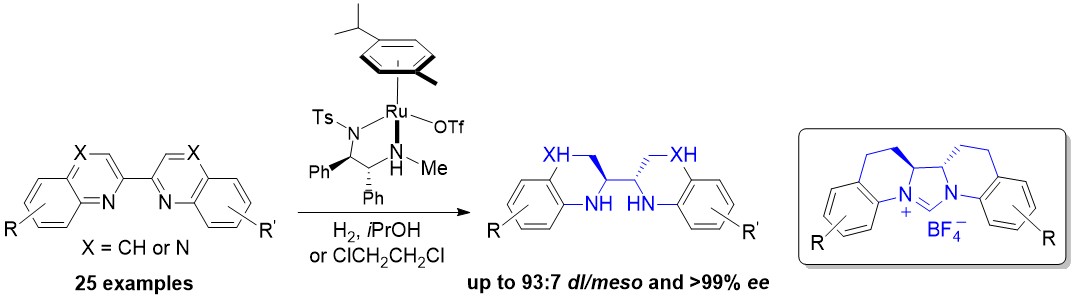
This work has been published in Angew. Chem. Int. Ed. 2016, 55, 12891-12894.

