Research Progress
-
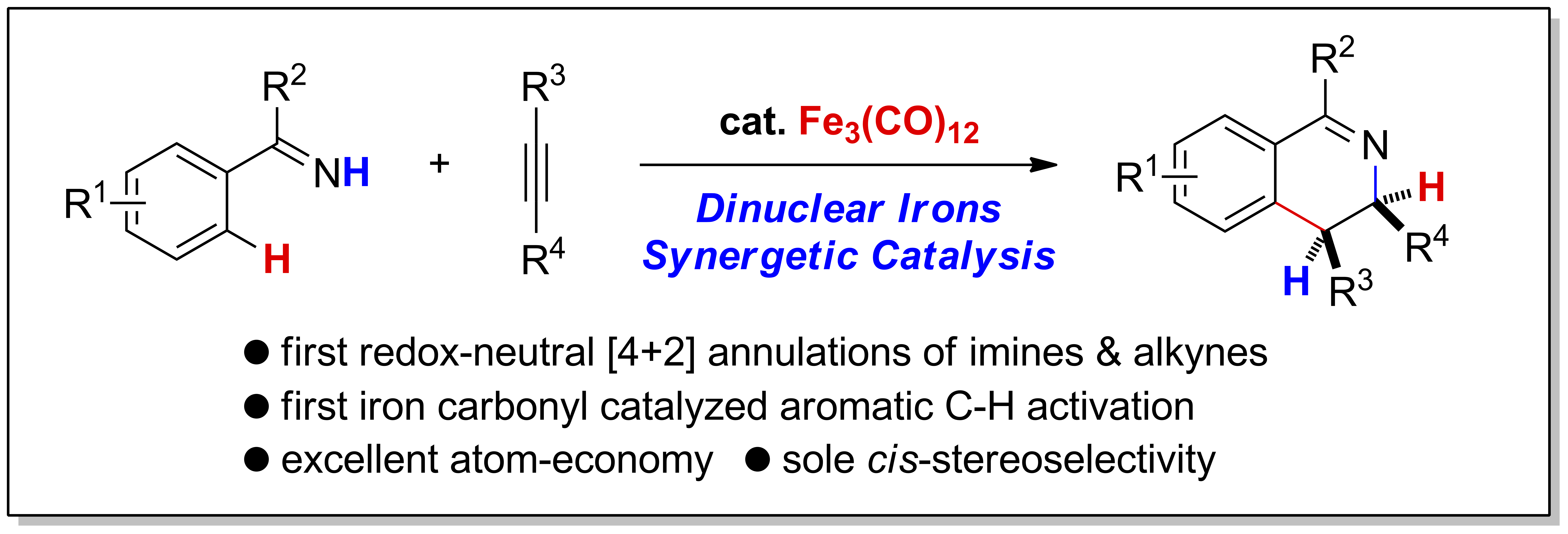
Iron Carbonyl Catalyzed Redox-Neutral [4+2] Annulation of N?H Imines and Internal Alkynes via C?H Bond Activation
Stoichiometric C?H bond activation of arenes mediated by iron carbonyls was reported by Pauson as early as in 1965, the catalytic C?H transformations based on which haven’t been developed yet. Herein, an iron-catalyzed annulation of N-H imines and internal alkynes to furnish cis-3,4-dihydroisoquinolines were described, which represents the first iron carbonyl catalyzed C?H activation reaction of arenes. Remarkablely, this is also the first redox-neutral [4+2] annulation of imines and alkynes vi ...
-
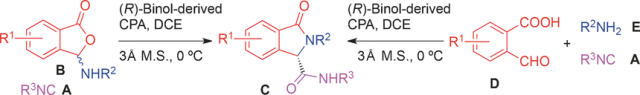
Chiral Phosphoric Acid Catalyzed Asymmetric Ugi Reaction by Dynamic Kinetic Resolution of the Primary Multicomponent Adduct
A novel triply interlocked [2]rotaxane based on a triptycene-derived tris(crown ether) host and a guest embedded with three dibenzylammonium and three N-methyltriazolium recognition sites was designed and synthesized, which showed pulley-like shuttling motion controlled by acid and base. Reaction of isonitriles with 3-(arylamino)isobenzofuran-1(3H)-ones in the presence of a catalytic amount of an octahydro (R)-binol-derived chiral phosphoric acid afforded 3-oxo-2-arylisoindoline-1-carboxamides i ...
-
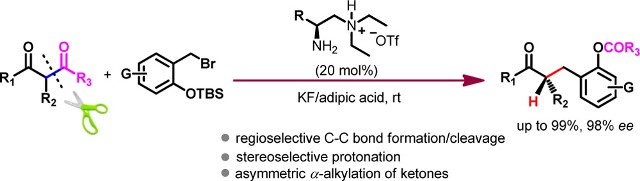
Asymmetric Retro-Claisen Reaction by Chiral Primary Amine Catalysis
The communication describes an enaminebased asymmetric retro-Claisen reaction of β-diketones by primary amine catalysis. The reaction proceeds via a sequence of stereoselective C?C formation, C?C cleavage, and a highly stereospecific enamine protonation to afford chiral α-alkylated ketones or macrolides with high yields and enantioselectivities. A detailed mechanism was explored on the basis of experimental evidence and computational studies to account for the observed stereocontrol.
-
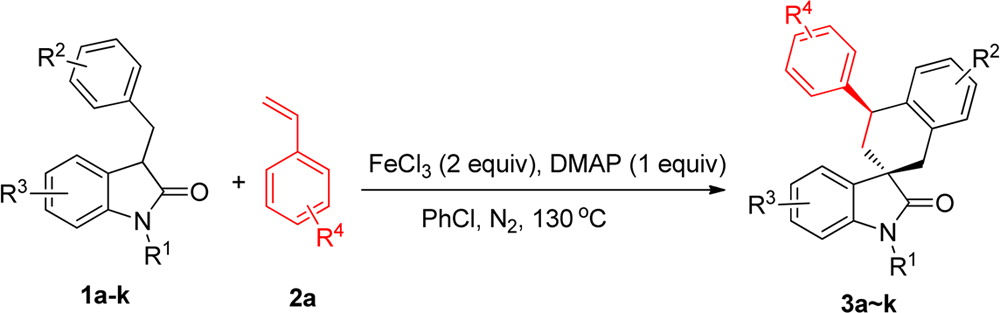
FeCl3?Mediated Radical Tandem Reactions of 3?Benzyl-2-oxindoles with Styrene Derivatives for the Stereoselective Synthesis of Spirocyclohexene Oxindoles
A novel FeCl3-mediated reaction of 3-benzyl-2-oxindoles with styrene derivatives was developed. The reaction provided spirocyclohexene oxindoles in good yields and excellent diastereoselectivities via a tandem radical addition cyclization process.
-
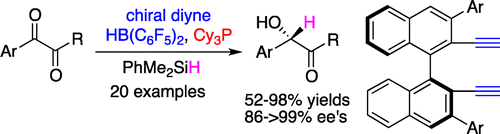
Chiral Frustrated Lewis Pairs Catalyzed Highly Enantioselective Hydrosilylations of 1, 2-Dicarbonyl Compounds
A highly enantioselective hydrosilylation of 1,2-dicarbonyl compounds was successfully realized for the first time utilizing the combination of tricyclohexylphosphine and chiral alkenylborane derived in situ from diyne as a frustrated Lewis pair catalyst. A variety of optically active α-hydroxy ketones and esters were obtained in 52–98% yields with 86–99% ee’s.
-
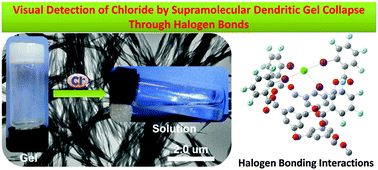
Halogen-Bonding for Visual Chloride Ion Sensing: A Case Study Using Supramolecular Poly(Aryl Ether) Dendritic Organogel Systems
A convenient and straightforward method for the visual recognition of chloride ion has been established through a chloride-responsive dendritic organogel. The specificity was largely attributed to the higher binding affinity of the dendritic gelator for chloride compared with other anions through halogen bonding interactions
-
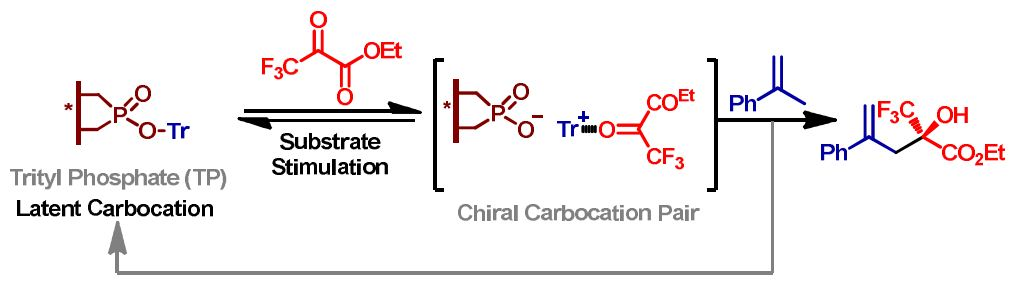
Asymmetric Latent Carbocation Catalysis with Chiral Trityl Phosphate
Stable carbocations such as tritylium ions have been widely explored as organic Lewis acid catalysts and reagents in organic synthesis. However, achieving asymmetric carbocation catalysis remains elusive ever since they were first identified over one century ago. The challenges mainly come from their limited compatibility, scarcity of chiral carbocations, as well as the extremely low barrier to racemization of chiral carbenium ions. We reported here a latent concept for asymmetric carbocation ca ...
-
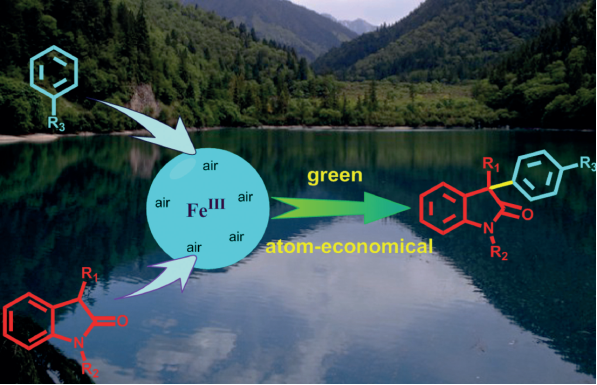
Fe(III)-Catalyzed Cross Dehydrogenative Arylation (CDA) between Oxindoles and Arenes under an Air Atmosphere
An efficient Fe(III)-catalyzed cross dehydrogenative arylation (CDA) of 3-substitueted-oxindoles with electron-rich aromatic and heteroaromatic compounds by using aerobic oxygen as an oxidant source was developed to provide 3,3’-disubstitueted-oxindoles in good yields.
-
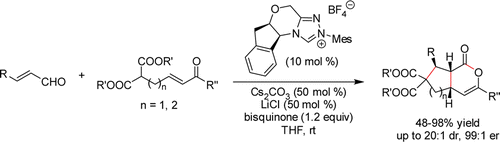
Enantioselective Synthesis of Bicyclic δ?Lactones via N?Heterocyclic Carbene- Catalyzed Cascade Reaction
The N-heterocyclic carbene-catalyzed cascade reaction of enals with malonates to give bicyclic δ-lactones was developed. The cyclopentane- and cyclohexane-fused δ-lactones with three continued stereocenters were obtained in high yields with excellent diastereo- and high enantioselectivities.
-
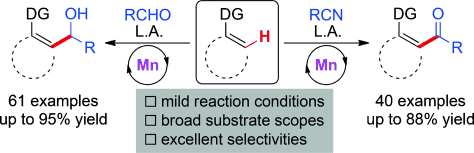
Manganese-Catalyzed Direct Nucleophilic C(sp2)?H Addition to Aldehydes and Nitriles
Grignard-type C?H nucleophilic addition to aldehydes and nitrles is a severe challenge for inert C?H bonds. Herein, a manganese-catalyzed nucleophilic addition of inert C(sp2)?H bonds to aldehydes and nitriles is disclosed by virtue of a dual activation strategy, namely, merging C?H activation by a manganese catalyst and aldehyde/nitrile activation by zinc bromide. The reactions feature mild reaction conditions, excellent regio- and stereoselectivity, and wide substrate scopes including both aro ...
-
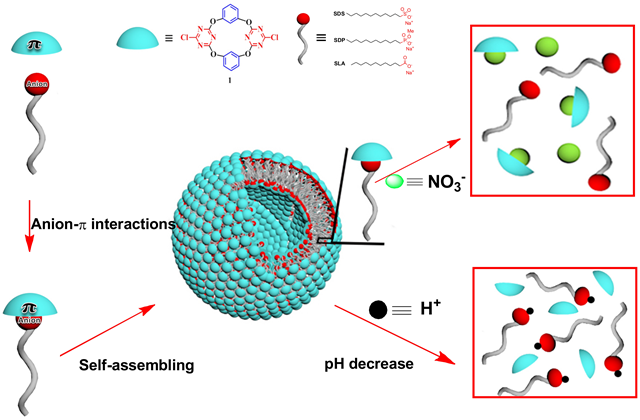
Self-assembly and disassembly of vesicles as controlled by anion-pi interactions
Anion-pi interactions have recently been widely studied as new non-covalent driving forces in supramolecular chemistry. However, anion-pi interactions induced self-assemblies are still largely unexplored. Herein we reported an unprecedented example of supramolecular amphiphiles formation driven by anion-pi interactions, and the subsequent self-assembled vesicles formation in water. By taking the p receptor 1 as the host, and anionic amphiphiles such as sodium dodecylsulfate (SDS), sodium laurate ...
-
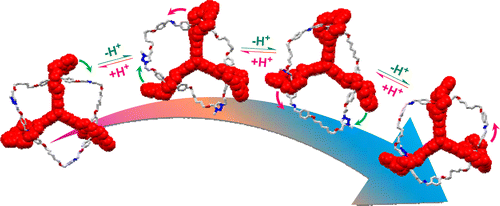
Stepwise Motion in a Multivalent [2](3)Catenane
The motions of biomolecular machines are usually multistep processes and involved in a series of conformational changes. In this paper, a novel triply interlocked [2](3)catenane composed of a tris(crown ether) host eTC and a circular ditopic guest with three dibenzyl ammonium (DBA) sites and three N-methyltriazolium (MTA) sites was reported. Due to multivalency nature of the catenane, the acid-base triggered motion was performed by a stepwise manner. The co-conformations of the four related stab ...
-
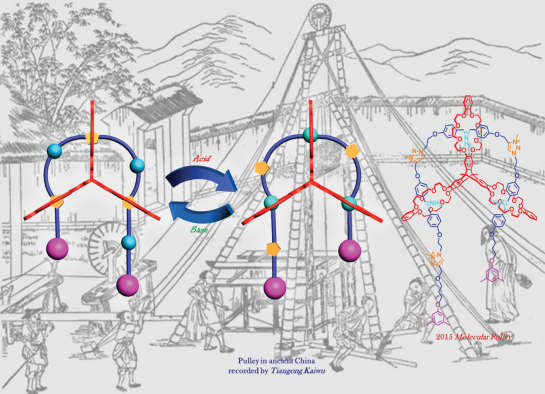
A Molecular Pulley Based on a Triply Interlocked [2]Rotaxane
A novel triply interlocked [2]rotaxane based on a triptycene-derived tris(crown ether) host and a guest embedded with three dibenzylammonium and three N-methyltriazolium recognition sites was designed and synthesized, which showed pulley-like shuttling motion controlled by acid and base.
-
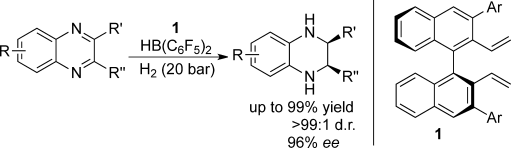
A Highly Cis-Selective and Enantioselective Metal-Free Hydrogenation of 2,3-Disubstituted Quinoxalines
The hydrogenation of 2,3-substituted quinoxalines belongs to one of the unsolved problems. Therefore, the development of highly stereoselective hydrogenations of 2,3-disubstituted quinoxalines with a good substrate scope, especially, the corresponding asymmetric reactions, is still a challenging subject. In this work, a wide range of 2,3-disubstituted quinoxalines has been successfully hydrogenated using borane catalysts to produce tetrahydroquinoxalines in 80-99% yields with excellent cis-selec ...
-
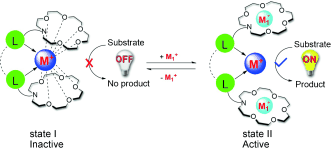
Cation-Triggered Switchable Asymmetric Catalysis with Chiral Aza-CrownPhos
An aza-crown ether, modified phosphoramidite ligand, has been designed and synthesized. The ON/OFF reversible switch of catalytic activity for its rhodium catalyst was thoroughly investigated in the asymmetric hydrogenation of dehydroamino acid esters modulated by host–guest interactions. In the OFF?state, the catalyst is almost inactive (less than 1?% conversion) because of the formation of an intermolecular sandwich complex by two aza-crown ether moities and the cationic rhodium metal center. ...