Chiral Phosphoric Acid Catalyzed Asymmetric Ugi Reaction by Dynamic Kinetic Resolution of the Primary Multicomponent Adduct
Yun Zhang, Yu-Fei Ao, Zhi-Tang Huang, De-Xian Wang,* Mei-Xiang Wang,* and Jieping Zhu*
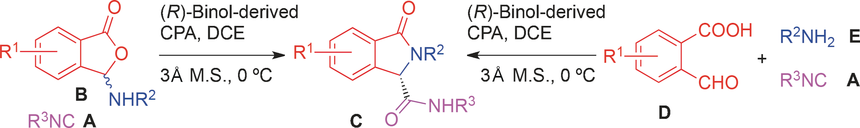
A novel triply interlocked [2]rotaxane based on a triptycene-derived tris(crown ether) host and a guest embedded with three dibenzylammonium and three N-methyltriazolium recognition sites was designed and synthesized, which showed pulley-like shuttling motion controlled by acid and base. Reaction of isonitriles with 3-(arylamino)isobenzofuran-1(3H)-ones in the presence of a catalytic amount of an octahydro (R)-binol-derived chiral phosphoric acid afforded 3-oxo-2-arylisoindoline-1-carboxamides in high yields with good to high enantioselectivities. An enantioselective Ugi fourcenter three-component reaction of 2-formylbenzoic acids, anilines, and isonitriles was subsequently developed for the synthesis of the same heterocycle. Mechanistic studies indicate that the enantioselectivity results from the dynamic kinetic resolution of the primary Ugi adduct, rather than from the C-C bond-forming process. The resulting heterocycle products are of significant medicinal importance.
This work has been published in Angew. Chem. Int. Ed., 2016, 55, 5282-5285.

