Asymmetric Retro-Claisen Reaction by Chiral Primary Amine Catalysis
Yunbo Zhu, Long Zhang and Sanzhong Luo*
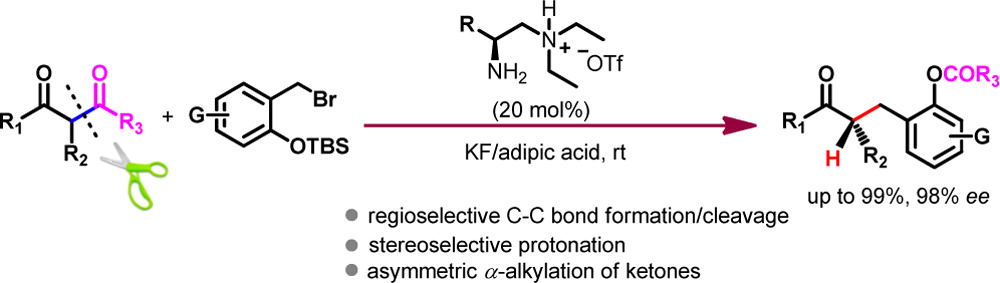
The communication describes an enaminebased asymmetric retro-Claisen reaction of β-diketones by primary amine catalysis. The reaction proceeds via a sequence of stereoselective C−C formation, C−C cleavage, and a highly stereospecific enamine protonation to afford chiral α-alkylated ketones or macrolides with high yields and enantioselectivities. A detailed mechanism was explored on the basis of experimental evidence and computational studies to account for the observed stereocontrol.
This work has been published in J. Am. Chem. Soc., 2016, 138, 3978-3981.

