Catalytic Asymmetric Mannich Reaction with N-Carbamoyl Imine Surrogates of Formaldehyde and Glyoxylate
Yang’en You, Long Zhang, Linfeng Cui, Xueling Mi, Sanzhong Luo *
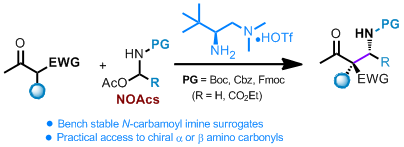
N, O-acetals (NOAcs) have been developed as bench stable surrogates for N-carbamoyl (Boc, Cbz and Fmoc) formaldehyde and glyoxylate imines in asymmetric Mannich reactions. The NOAcs can be directly utilized in the chiral primary amine catalyzed Mannich reactions of both acyclic and cyclic β-ketocarbonyls with high yields and excellent stereoselectivity. The current reaction offers a straightforward approach in the asymmetric synthesis of α- or β-amino carbonyls bearing chiral quaternary centers in a practical and highly stereocontrolled manner.
This work has been published in Angew. Chem. Int. Ed. 2017, 56, 13814-13818.

