Dichotomy of Manganese Catalysis via Organometallic or Radical Mechanism: Stereodivergent Hydrosilylation of Alkynes
Xiaoxu Yang and Congyang Wang*
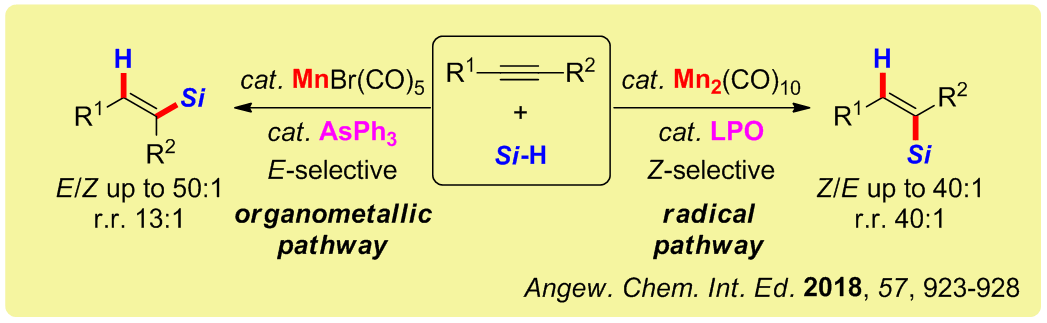
Herein, we disclose the first manganese‐catalyzed hydrosilylation of alkynes featuring diverse selectivities. The highly selective formation of E‐products was achieved by using mononuclear MnBr(CO)5 with the arsenic ligand, AsPh3. Whereas using the dinuclear catalyst Mn2(CO)10 and LPO (dilauroyl peroxide) enabled the reversed generation of Z‐products in good to excellent stereo‐ and regioselectivity. Such a way of controlling the reaction stereoselectivity is unprecedented. Mechanistic experiments revealed the dichotomy of manganese catalysis via organometallic and radical pathways operating in the E‐ and Z‐selective routes, respectively.
This work has been published in Angew. Chem. Int. Ed. 2018, 57, 923-928.

