Aromatic C-H Addition of Ketones to Imines Enabled by Manganese-Catalysis
Bingwei Zhou, Yuanyuan Hu, Ting Liu, and Congyang Wang*
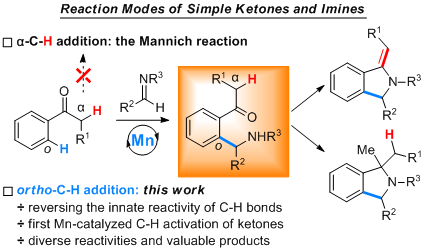
Selectivity control of varied C-H bonds in a complex molecule is a long-standing goal and still a great challenge in C-H activation field. Most often, such selectivity is achieved by the innate reactivity of different C-H bonds. In this context, the classic Mannich reaction of acetophenone derivatives and imines is ascribed to the more reactive C(sp3)-H bonds α to the carbonyl, with the much less reactive aromatic C(sp2)-H bonds remaining intact. Herein we report an aromatic C(sp2)-H addition of ketones to imines enabled by manganese-catalysis, which totally reverses the innate reactivity of C-H bonds α to the carbonyl and those on the aromatic ring. Diverse products of ortho-C-H aminoalkylated ketones, cyclized exo-olefinic isoindolines and three-component methylated isoindolines can be successfully accessed under mild reaction conditions, which significantly expands the synthetic utilities of ketones as simple bulk chemicals.
This work has been published in Nat. Commun. 2017, 8, 1169-1177.

