Frustrated Lewis Pairs Catalyzed Asymmetric Metal-Free Hydrogenations and Hydrosilylations
Wei Meng, Xiangqing Feng, and Haifeng Du*
The use of frustrated Lewis pairs is an extremely important approach to metal-free hydrogenations. In contrast to the rapid growth of catalytic reactions, asymmetric hydrogenations are far less developed due to a severe shortage of readily available chiral frustrated Lewis pair catalysts with high catalytic activities and selectivities. Unlike the stable Lewis base component of frustrated Lewis pairs, the moisture-sensitive boron Lewis acid component is difficult to prepare. The development of convenient methods for the quick construction of chiral boron Lewis acids is therefore of great interest.
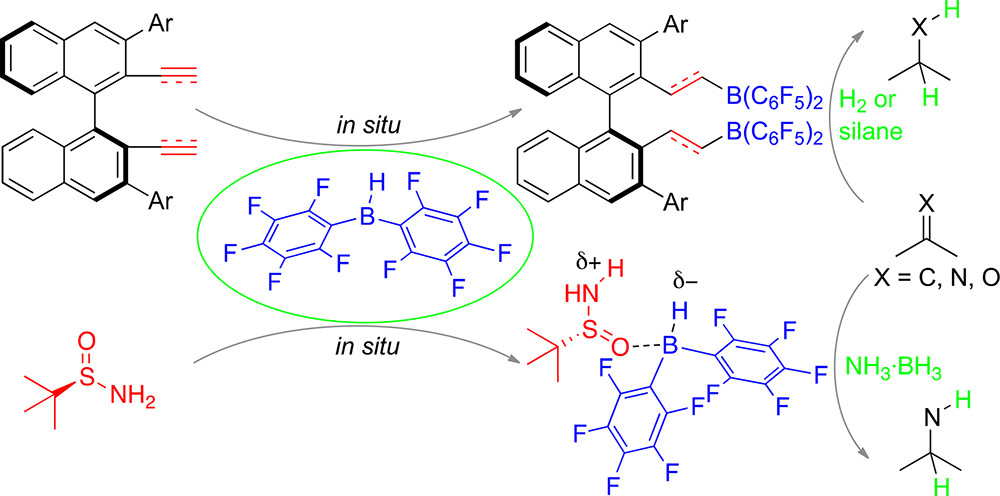
In this Account, we summarize our recent studies on frustrated Lewis pair-catalyzed, asymmetric metal-free hydrogenations and hydrosilylations. To address the shortage of highly active and selective catalysts, we developed a novel strategy for the in situ preparation of chiral boron Lewis acids by the hydroboration of chiral dienes or diynes with Piers’ borane without further purification, which allows chiral dienes or diynes to act like ligands. This strategy ensures the construction of a useful toolbox of catalysts for asymmetric metal-free hydrogenations and hydrosilylations is rapid and operationally simple. Another strategy is using combinations of readily available Lewis acids and bases containing hydridic and acidic hydrogen atoms, respectively, as a novel type of frustrated Lewis pairs. Such systems provide a great opportunity for using simple chiral Lewis bases as the origins of asymmetric induction.
With chiral diene-derived boron Lewis acids as catalysts, a broad range of unsaturated compounds, such as imines, silyl enol ethers, 2,3-disubstituted quinoxalines, and polysubstituted quinolines, are all viable substrates for asymmetric metal-free hydrogenations and give the corresponding products in good yields with high enantioselectivities and/or stereoselectivities. These chiral catalysts are very effective for bulky substrates, and the substrate scope for these metal-free asymmetric hydrogenations has been dramatically expanded.
Chiral alkenylboranes were designed to enhance the rigidity of the framework and modify the Lewis acidity through the resulting double bonds. Frustrated Lewis pairs of chiral alkenylboranes and phosphines are a class of highly effective catalysts for asymmetric Piers-type hydrosilylations of 1, 2-dicarbonyl compounds, and they give the desired products in high yields and enantioselectivities. Moreover, asymmetric transfer hydrogenations of imines and quinoxalines with ammonia borane as the hydrogen source have been achieved with frustrated Lewis pair of Piers’ borane and (R)-tert-butylsulfinamide as the catalyst. Mechanistic studies have suggested that the hydrogen transfer occurs via an 8-membered ring transition state, and regeneration of the reactive frustrated Lewis pair with ammonia borane occurs through a concerted 6-membered ring transition state.
This work has been published in Acc. Chem. Res., 2018, 51, 191–201.

